cGMP CONTRACT MANUFACTURING & CDMO Services
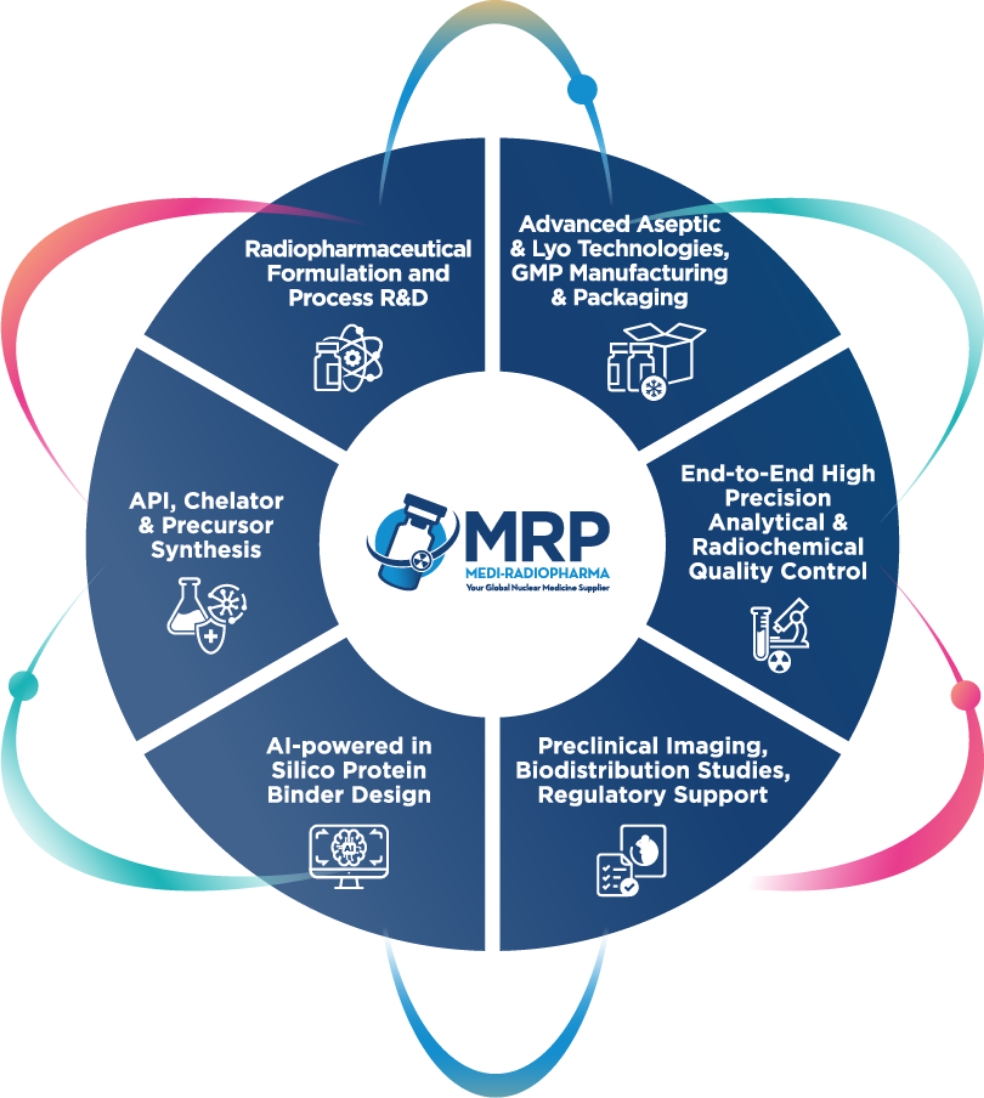
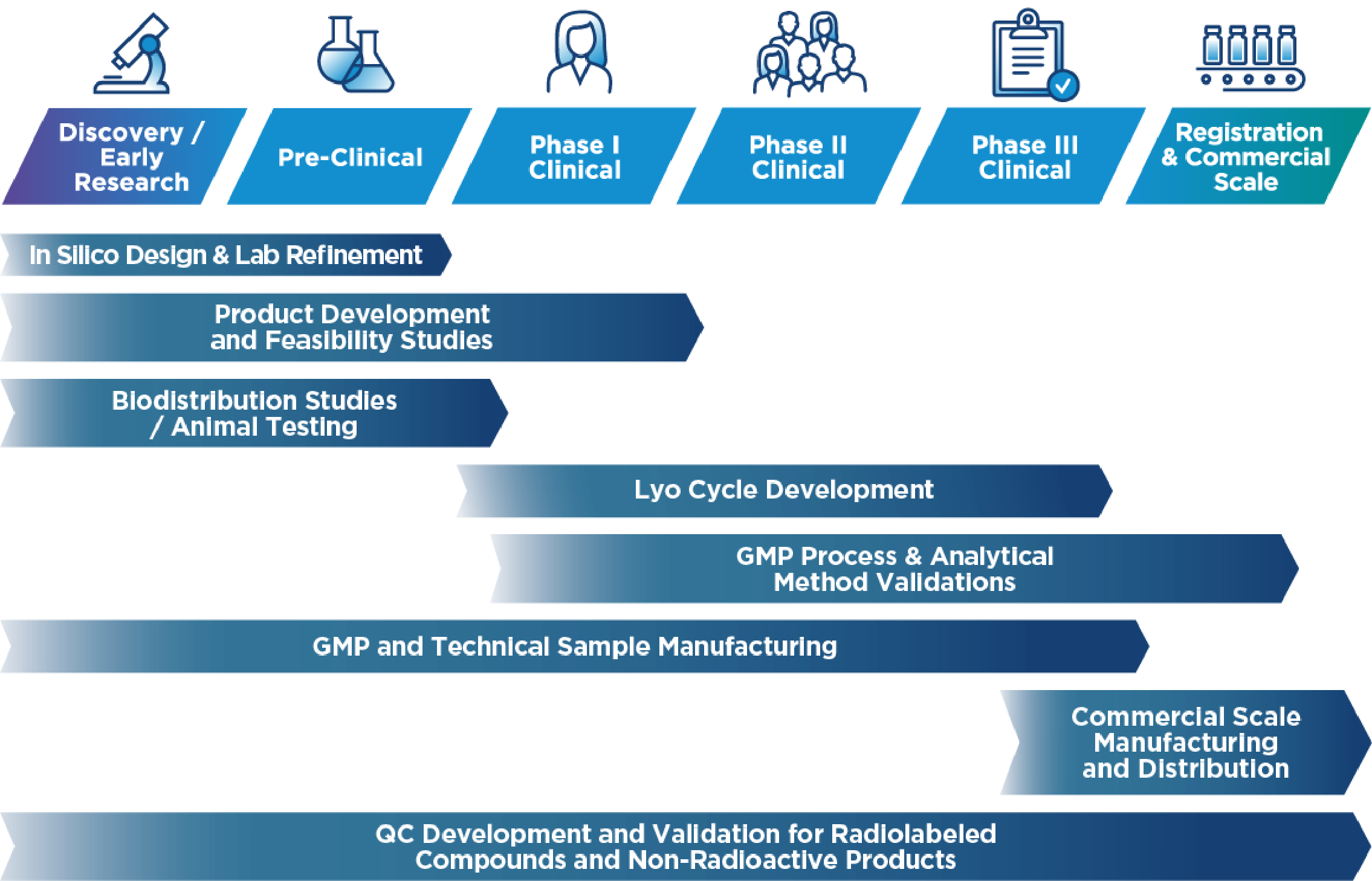
As a GMP-certified contract development and manufacturing organization (CDMO), we offer end-to-end solutions tailored to the needs of the pharmaceutical and nuclear medicine industries. Our expertise extends the entire development and production lifecycle, ensuring the highest quality and compliance standards.
- CDMO Services for Liquid & Lyophilized Products – Scalable batch production from 1 to 300 liters
- Sterile Small-Volume Manufacturing – cGMP-compliant production for clinical trials and R&D
- API Synthesis – Small molecule API synthesis under strict GMP compliance
- Drug Product Development – QbD-driven formulation, process development, validation, and scale-up for liquid and lyophilized injections
- All-Isotope License – Including α-Emitters, supporting a broad range of nuclear medicine applications
- Comprehensive Tech Transfer & Validation – Covering microbiological, analytical, radioanalytical controls, and stability testing
With a commitment to innovation and excellence, our experienced team provides flexible, scalable, and fully compliant manufacturing solutions to support your development and commercialization needs.
Let’s bring your projects to life—partner with us!